
MoCA provides a mechanism for European countries to collaborate on coordinated access to orphan medicines in a voluntary, dialogue-based approach, intended to create a fluid set of interactions between key stakeholders, across all aspects of a product. The roles, responsibilities and prerogatives of each of the participating stakeholders should be respected.
It is important to stress that, to date, nowhere else in Europe does such a platform exist where companies’ issues around reimbursement or financing schemes can be discussed with a variety of jurisdictions and societal perspectives.

This paper reflects the lessons learned from 10 years’ multi-stakeholder dialogue on improving access in Europe via MoCA.
Objectives of MoCA Pilots
Pilot projects retained within MoCA aim at facilitating “early dialogue” between companies and national competent authorities for pricing and reimbursement on specific orphan medicines, focusing discussions on a company’s strategy, the targeted disease, the product and its development, the approach and identified challenges to access markets with a view to ultimately helping to speed up access for patients in EU Member States to the product in question.
For further reference
- MoCA Revised Terms of Reference (January 2016)
- Timelines for MoCA Activity
- Glossary of Activities & Agencies with Potential Relevance for MoCA
- Points to Consider for Companies considering a pilot
Participants
Participation in MoCA is open to several stakeholder groups, including:
- National competent authorities for pricing and reimbursement
- Rare disease patients
- Candidate marketing authorisation applicant/holders willing to be involved in a pilot focused on a particular product of theirs
- European Medicines Agency and EunetHTA as observers
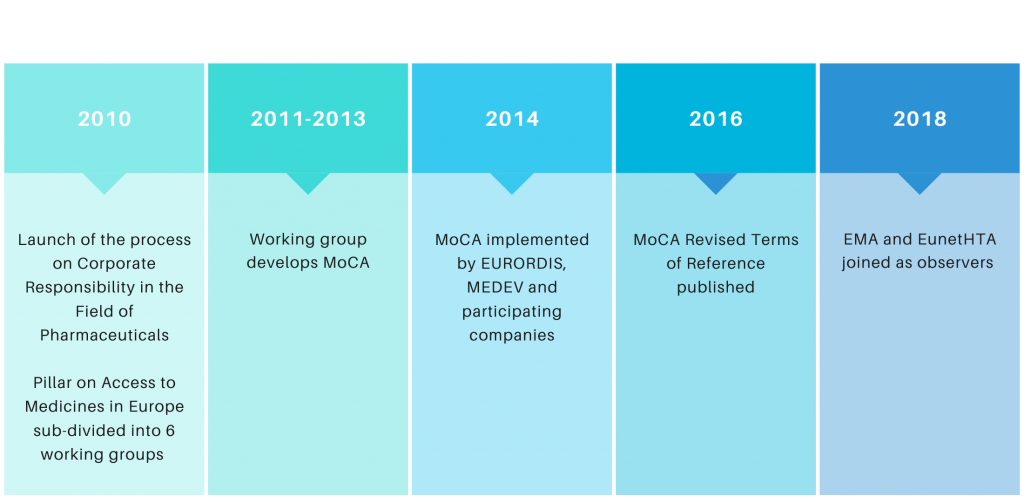
History
MoCA takes its roots in the “Process on Corporate Responsibility in the Field of Pharmaceuticals” launched under the Belgian Presidency in September 2010, which was intended to identify areas for improved cooperation in the European Commission’s Communication on Industrial Policy due for publication in late 2012.
For further reference
- Corporate responsibility in the pharmaceutical industry
- Corporate Responsibility in the field of Pharmaceuticals
Transparent Value Framework
The Process on Corporate Responsibility consisted of 3 pillars, the first of which was “Access to Medicines in Europe”. That pillar itself was subdivided from the beginning into 6 different working groups, the second of which focused on the issue of “coordinated access to orphan medicinal products”. The formation of that working group marked the creation of MoCA.
In its original form, MoCA was intended to map innovative ways to provide real-life access to orphan medicines, in an affordable and sustainable way, for patients with unmet medical needs for whom these solutions would otherwise be out of reach. The main recommendation of the group was to develop a coordinated mechanism between volunteering Member States and orphan medicine developers to evaluate the value of an orphan medicinal product, which could be based on a transparent value framework, in order to support the exchange of information aimed at enabling informed decisions at Member State level on pricing and reimbursement. This, ultimately, should lead to more rational prices for payers, more predictable market conditions for industry and more equitable access for patients.
After the group completed their work in 2013, a few working group members decided to pursue discussions under the umbrella of the Medicines Evaluation Committee (MEDEV, an informal group of experts from statutory health insurance institutions and HTA agencies in Europe) with a view to putting all conclusions and recommendations into practice through the implementation of pilot projects focused on actual orphan medicines in development.
For further reference
- Working Group on Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA-OMP): Final Report
- Working Group on Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA-OMP): Key Conclusions and Recommendations
- Working Group on Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA-OMP): Transparent Value Framework
- The Medicines Evaluation Committee (MEDEV)
Presentations about MoCA
2019
2018
2017
2016
2015
- Access, the Key to Successful European Commercialisation (Wills Hughes-Wilson)
- Early Dialogue between Payers and Developers (Anna Bucsics)
- Early Dialogue between Payers and Developers (Yann Le Cam)
2014
- MoCA Concept and Pilot Project (Wills Hughes-Wilson)
- MoCA (Ri De Ridder)
- Spending Wisely (Anna Bucsics)
2013
- Corporate Responsibility in Improving Access to OMPs (Thomas Heynisch)
- Common European Transparent Value Framework (Flaminia Macchia)
- EMA-HTA Parallel Scientific Advice (Yann Le Cam)
2010
Articles about MoCA
2023
2020
2014
2012
Documents with References to MoCA
- EURORDIS-European Patients’ Forum (EPF) Joint Call on EU National Competent Authorities for Pricing & Reimbursement
- Implementation report on the Commission Communication on Rare Diseases: Europe’s
challenges [COM(2008) 679 final] and Council Recommendation of 8 June 2009 on an action in the field of rare diseases (2009/C 151/02) - 2014 Report on the State of the Art of Rare Diseases Activities in Europe
For any question, please contact:
Anna Bucsics, MoCA Project Advisor
Šárka Kubinová, MoCA Project Coordinator
MoCA.OMP@gmail.com
Maria Cavaller, Patient Engagement & Therapeutic Development Senior Manager
maria.cavaller@eurordis.org
