Bringing together 300+ patient representatives from 28 European countries, the European Patient Advocacy Groups (ePAGs) represent the patient voice in the European Reference Networks (ERNs).
ePAG essentials
In 2017, following years of advocacy efforts from the rare disease community and EURORDIS, the European Commission launched 24 European Reference Networks (ERNs) specialised in rare or low-prevalence complex diseases, which provided an unprecedented capacity for cross-border collaboration to diagnose and treat those diseases.
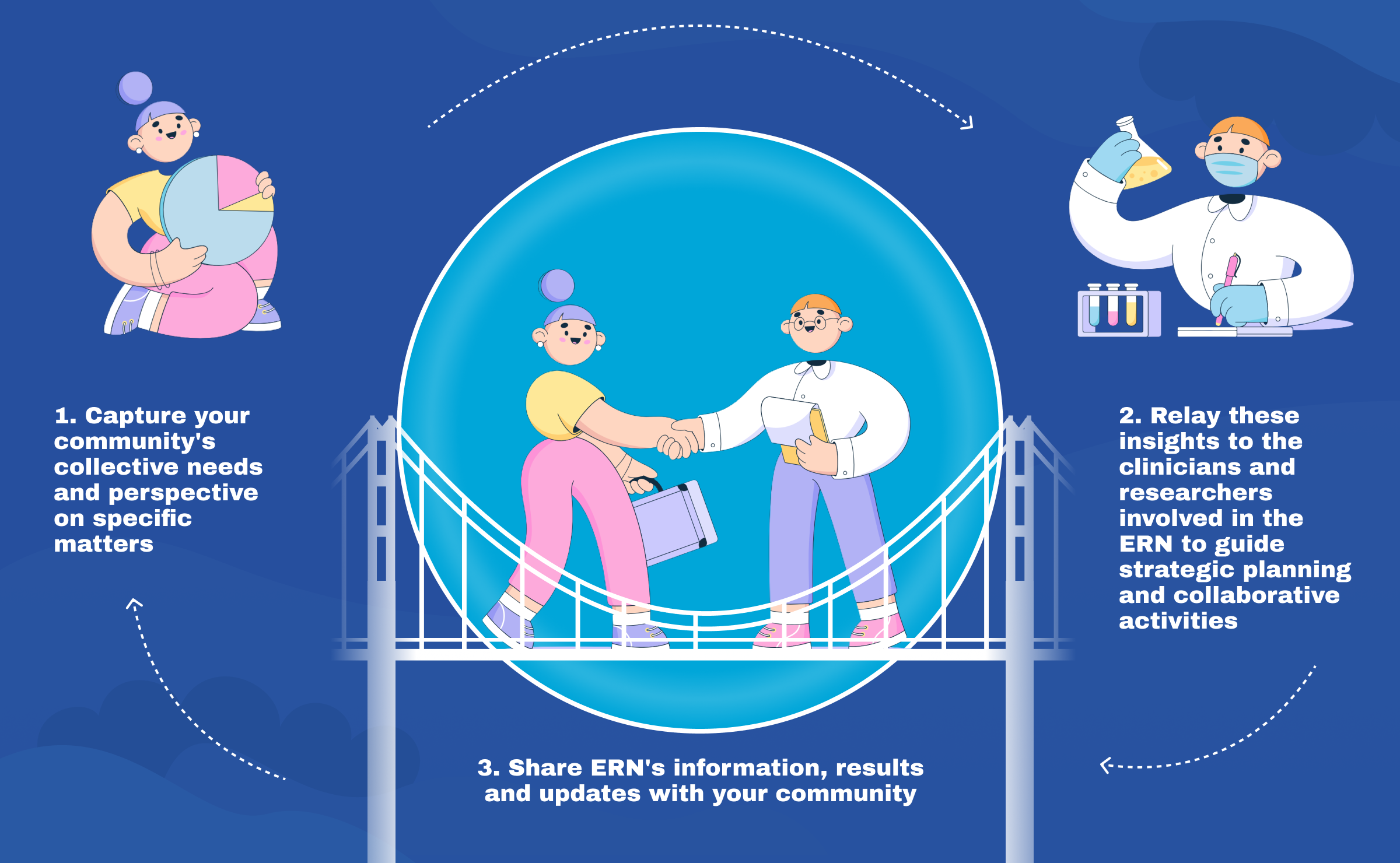
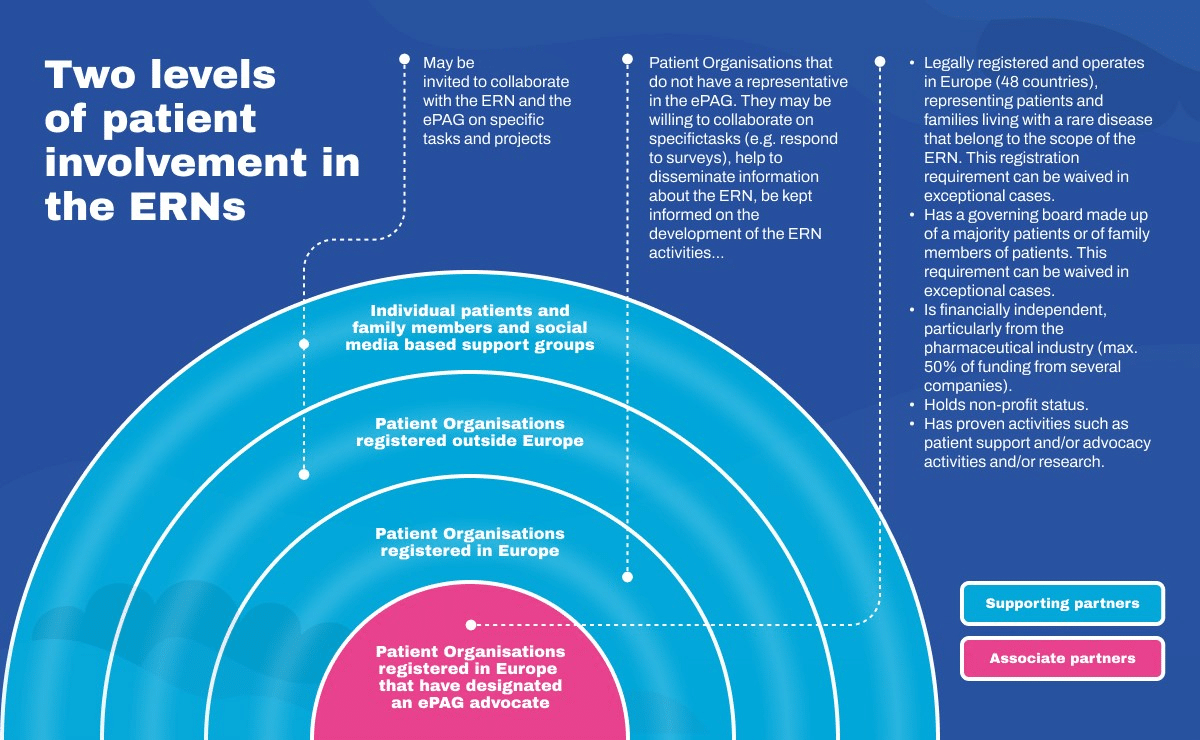
In parallel, EURORDIS together with the rare disease community launched the European Patient Advocacy Groups (ePAGs) to bring the patient voice to the heart of the ERNs’ activities. Each of the 24 ePAGs, one per ERN, brings together rare disease patient organisations and advocates who are actively involved in the ERNs, working in partnership with clinicians and researchers.
If you want to learn more about the ePAGs and their activities, please visit their webpages below:
-
List of European Patient Advocacy Groups (ePAGs)