The growing number of rare diseases lacking treatment is an important public health issue. Often the scarcity of incentives for drug manufacturers and the lack of evidence supporting the applications limit the number of new orphan drugs that ultimately access the market.
The growing number of rare diseases lacking treatment is an important public health issue. Often the scarcity of incentives for drug manufacturers and the lack of evidence supporting the applications limit the number of new orphan drugs that ultimately access the market.
Orphan medicines legislation
Orphan medicines legislation provides incentives to pharmaceutical companies to develop and market medicinal products to treat rare diseases.
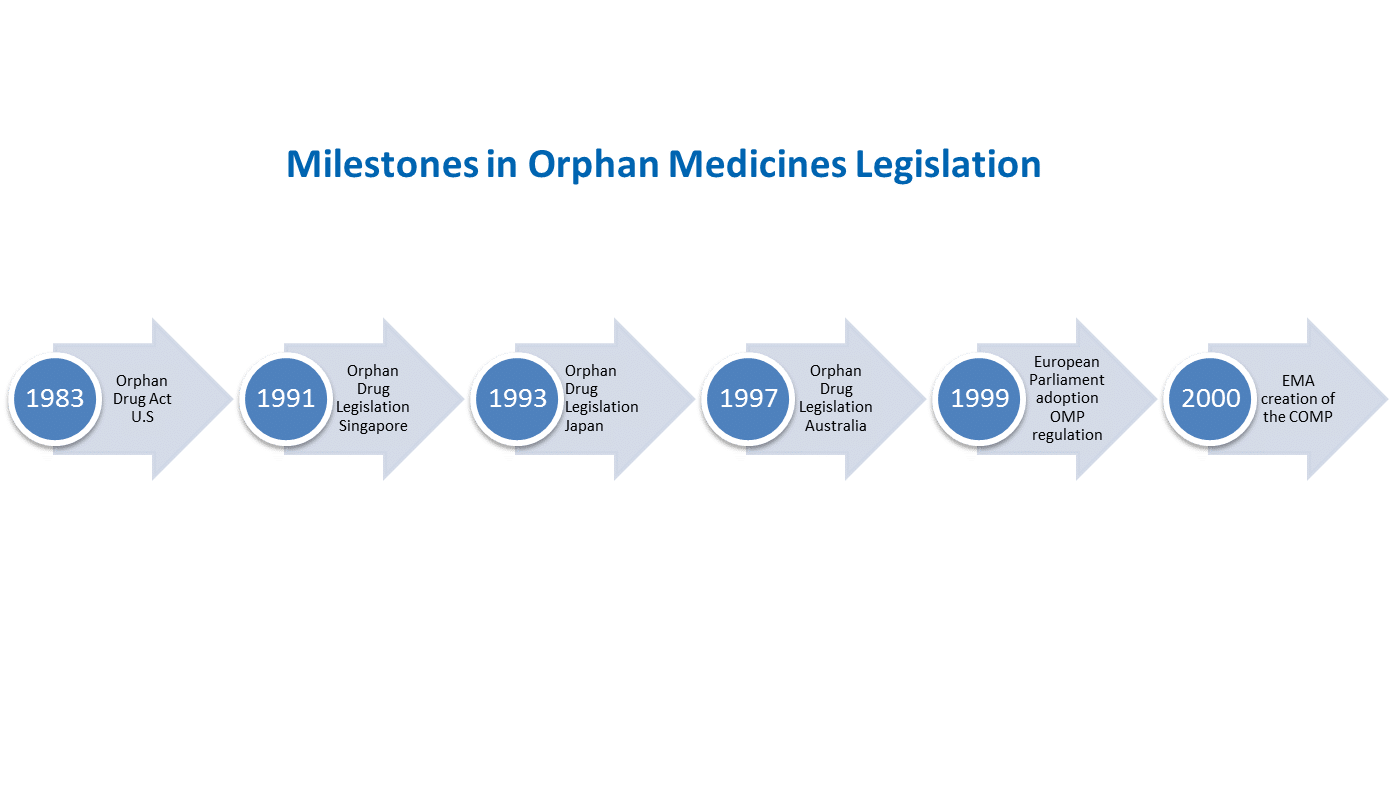
A ground-breaking precedent was set by the United States in 1983 when the Orphan Drug Act came into effect, which was followed by Japan and Australia in the 1990s. In Europe, the European Parliament adopted the EU Regulation on Orphan Medicinal Products (Regulation EC n° 141/2000) on 16 December 1999. EURORDIS, representing the rare disease patient community in Europe, actively advocated for the adoption of this legislation.
Following the adoption of the regulation, the Committee for Orphan Medicinal Products (COMP) was created within the European Medicines Agency (EMA) to review the applications for medicinal products seeking an orphan designation.
Incentives provided by the EU Regulation
-
Market exclusivity in the EU:
When an orphan medicinal product (OMP) receives marketing authorisation benefits from 10 years of market exclusivity, which protects them from market competition from similar products. For paediatric OMPs this period is extended to 12 years.
- Protocol assistance:
The EMA provides scientific advice for OMPs (protocol assistance) at no cost or at a reduced fee to optimise the development of OMPs and to ensure better compliance with EU regulatory requirements.
- Fee reductions:
During the approval process, fee waivers for orphan designation and reduced fees are granted. These apply to marketing authorisation, inspections, variations and protocol assistance.
- EU-funded research:
Pharmaceutical companies developing orphan drugs may be eligible for specific grants from EU and Member State programmes as well as initiatives supporting research and development. This includes the Community framework programmes.
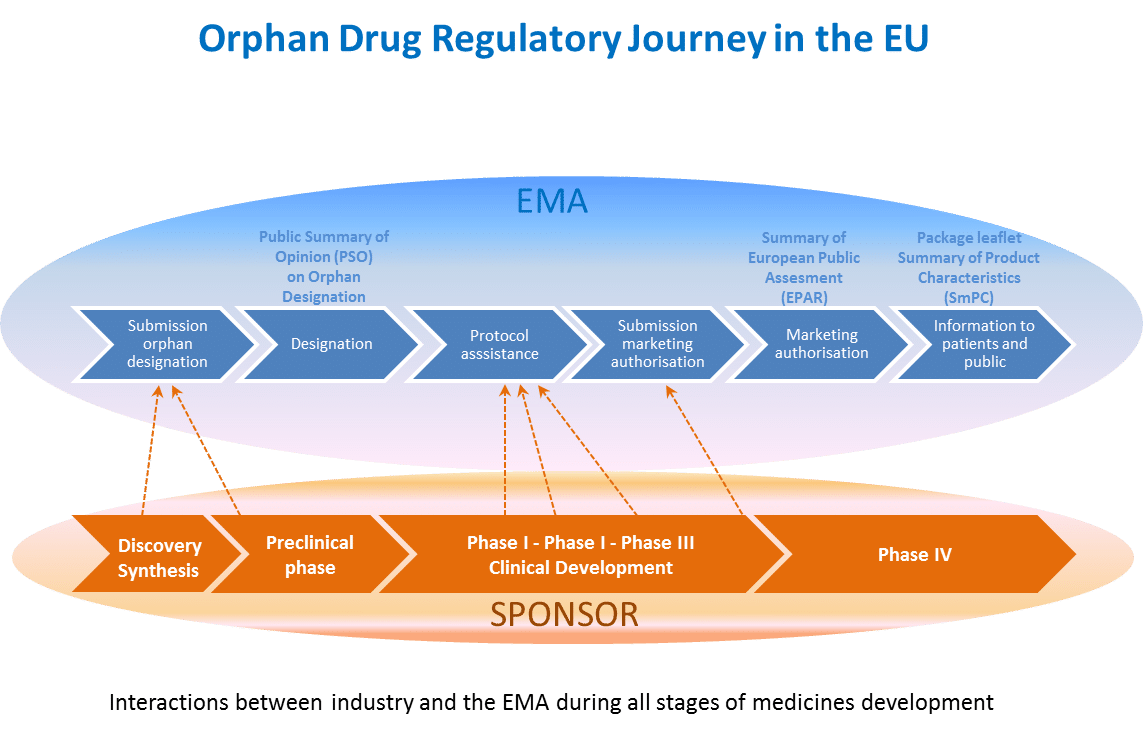
The following graph shows all the stages of the centralised procedure for OMPs in Europe.