ERDERA 101: Europe’s New Chapter in Rare Disease Research
October 2024Europe is home to over 30 million people living with a rare disease, yet healthcare systems remain ill-equipped to meet their complex needs. Of the more than 7,000 known rare conditions, a staggering 94% have no dedicated treatment, and one in three rare disease patients has never received a therapy tailored to their condition[i].
Despite these challenges, participation in research remains limited: only 18% of rare disease patients have contributed to research on treatments[ii], and just 15% have been involved in quality-of-life studies[iii].
Addressing these gaps in care and research is where the newly launched European Rare Diseases Research Alliance (ERDERA) comes in.
Welcomed by EURORDIS-Rare Diseases Europe last month, ERDERA is the largest co-funded partnership ever established in the field of rare disease research and innovation. With an estimated budget of €385.5 million, it is aiming to unify Europe’s fragmented research efforts under a single framework to accelerate the development of diagnostics and therapies.
But what are the origins of ERDERA, how is it structured and funded, and what are its key goals? And, crucially, what role will patients and patient organisations play in ensuring it supports research that truly addresses the unmet needs of the rare disease community?
We break down everything that Europe’s rare disease community needs to know about this milestone research partnership.
The Origins: ERDERA’s roots and inception
ERDERA builds on over two decades of EU-led efforts to strengthen rare disease research and collaboration.
ERDERA’s direct predecessor, the European Joint Programme on Rare Diseases (EJP RD), brought together over 130 institutions from 35 countries and laid the foundations for a coordinated research environment. Through shared data infrastructures, research funding, and the integration of patient perspectives, the EJP RD made significant progress in enhancing cooperation across the field.
However, the EJP RD also highlighted the need for a more comprehensive and sustainable approach to tackle ongoing fragmentation, namely in clinical research.
This is what has birthed the creation of ERDERA, which is endeavouring to address these challenges by engaging a larger and more diverse group of stakeholders, prioritising patient-driven research, and providing a robust framework to support long-term advancements in diagnostics and treatments.
The Mission: ERDERA’s goals and ambitions
ERDERA’s mission is to reduce the average time to diagnosis, increase the availability of therapeutic options, and ensure that rare disease research is sustainable and patient-driven.

By bringing together 178 organisations from 37 countries from the public and private sectors, including 150 partners from 25 EU Member States under Horizon Europe, ERDERA seeks to foster a collaborative environment where innovations can thrive.
Key objectives of ERDERA include:
- Bring under one roof a broad range of high-value services, resources and cross-disciplinary expertise to support rare disease research;
- Boost clinical research by enabling every consenting patient living with a rare disease to be findable and enrolled in a suitable clinical study;
- Spur innovation and EU competitiveness, through investment but also by aligning regional, national and European research strategies, and fostering collaboration among all stakeholders at a global scale
By addressing these priorities, ERDERA aims to position Europe as a global leader in rare disease research and innovation.
The objective really of ERDERA is to really finalise, if I can say so, the building of the rare diseases research ecosystem.
Dr. Daria Julkowska, Coordinator, European Rare Diseases Research Alliance
The Plan: ERDERA’s structure, funding, and approach
With a projected budget of €385.5 million over seven years (2024-2031), co-funded through the EU’s Horizon Europe programme, national Member States, countries associated to Horizon Europe, and in cash and in-kind contributions from public and private partners, ERDERA’s activities are organised around four main pillars encompassing 25 interconnected work packages:
- Inter(national) Capacity Alignment: Aligns research strategies across Europe and beyond, ensuring all countries can participate. ERDERA will ensure alignment between national and international rare disease research strategies, particularly in nations that are behind in developing and implementing national plans.
- Rare Disease Research Funding: The financial backbone providing grants to support collaborative research projects, clinical trials across Europe as well as knowledge exchange and networking initiatives.
- Clinical Research Network (CRN): Connects hospitals, clinics, and researchers to improve the diagnosis and treatment of rare diseases. This network will enhance diagnostics and clinical trial readiness, help assess the impact of rare diseases and support the development of advanced therapies. It builds upon the clinical expertise of the 24 European Reference Networks (ERNs).
- Support Services: Those support services encompass a Data Support Hub to facilitate and speed up data collection, integration analysis and sharing; an Expertise Services Hub to provide guidance on regulatory requirements, methodologies, and best practice in translational and clinical research; an Education & Training Hub to offer educational programmes and resources for patients, researchers, and clinicians; and an Acceleration Hub to speed up and advance the most promising research projects and solutions through collaboration between research teams and industry.
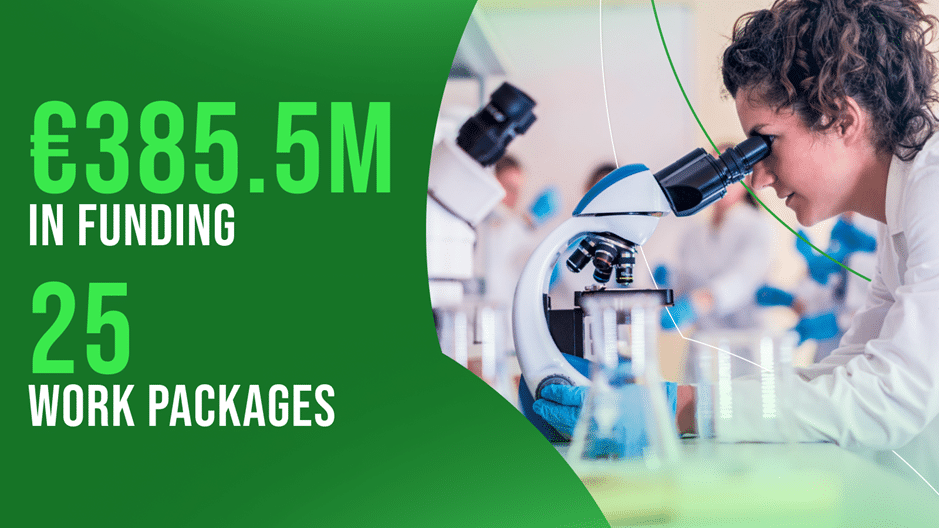
Together, these pillars form a comprehensive structure to drive ERDERA’s mission of transforming rare disease research across Europe.
With its 25 interconnected work packages, ERDERA covers everything needed for rare disease research – funding, data sharing, clinical research and trials, and even patient education. It’s all about working together to speed up progress and make a real difference for patients across Europe.
Dr. Holm Graessner, Managing Director, Center for Rare Diseases in Tübingen and Co-Leader of CRN in ERDERA
Patients’ Role: ERDERA involving our community
Patients and patient organisations are at the heart of ERDERA’s research strategy. As a key partner, EURORDIS plays a pivotal role in ensuring that patients’ perspectives shape research priorities and drive meaningful change.
EURORDIS is leading and participating in several work packages dedicated to patient engagement, education, and training. These include packages relating to initiatives such as the next Open Academy Schools, set to take place in Barcelona from 2-5 June 2025, which are continuing to accept applications from patient advocates until 26 October 2024.
By collaborating closely with other stakeholders, EURORDIS is creating a research environment where patient needs are prioritised in every step of the process.
ERDERA marks a turning point for rare disease research – bringing together science, innovation, and patient voices to drive meaningful change. By joining forces across borders and fields, we can make real breakthroughs that will transform lives and show what’s truly possible. Having patients involved at every step isn’t just a priority; it’s what will make ERDERA’s work truly matter.
Dr. Roseline Favresse, Research Policy and Initiatives Director, EURORDIS
Getting involved!
Patient representatives and the rare disease community will play a vital role in shaping ERDERA’s future. A PPIE (Patient and Public Involvement and Engagement) group has been structured and is embedded throughout ERDERA. Led by EURORDIS, it will be co-driven by World Duchenne Organisation, AFM-Téléthon, Rare Diseases International, Genetic Alliance UK and Health Research Charities Ireland. This group will guide engagement practices and ensure patients’ needs are prioritised. It will also help onboard patients in the different ERDERA activities.
National Mirror Groups (NMGs) will serve as platforms for engaging with national stakeholders and aligning country-specific actions with ERDERA’s broader European goals. These groups will empower rare disease national alliances to take on leadership roles and influence research and policy decisions at national and EU levels. Supported by dedicated funding, NMGs will help patient advocates translate EU strategies into national programmes.
Additional opportunities for patient advocates will include joining working groups, participating in clinical research, or engaging in educational programmes like our 2025 Open Academy Schools.
As we prepare for the partnership’s launch in Paris on 28 October, stay connected by subscribing to ERDERA’s newsletter and following EURORDIS’ own communication channels.
The involvement of the rare disease community will be crucial in driving research that truly transforms the healthcare of those living with under-treated and under-researched conditions.
Julien Poulain, Communications Manager
[i] EURORDIS (n.d.) More available, accessible, and affordable treatments.
[ii] EURORDIS (2021) Recommendations from the Rare 2030 Foresight Study: The Future of Rare Diseases Starts Today, p. 95.
[iii] Ibid., p.119.