Health Technology Assessments in 2025: What changes are coming?
December 2024On 12 January 2025, the European healthcare landscape will reach a pivotal milestone with the application of the EU Health Technology Assessment (HTA) Regulation.
This new framework will revolutionise how new medicines and health technologies are evaluated across the European Union, fostering greater collaboration, efficiency, and inclusivity. For Europe’s rare disease community, the Regulation holds significant promise, offering the potential to accelerate access to much-needed treatments across Member States.
But what exactly will change from 12 January, and how will the new Regulation impact patients, the industry, healthcare professionals, and policymakers alike?
Given the crucial role HTAs play in determining access to treatments for rare diseases, EURORDIS-Rare Diseases Europe delves into the details of this transition to help our community understand what lies ahead.
What are Health Technology Assessments and why do they matter?
Health Technology Assessment (HTA) is a multidisciplinary approach that compares a new technology with an already existing one to assess whether it is more, equally, or less effective. HTA helps national authorities decide which treatments offer the best value for healthcare systems and the patients they serve. By evaluating medical, social, economic, and ethical factors, HTAs guide decisions on which healthcare practices and technologies should be prioritised.
HTAs differ from marketing authorisation processes. While marketing authorisation ensures a product is safe, effective, and of high quality (allowing it to be marketed), HTAs evaluate whether the product is worth funding and drive national reimbursement decisions. They consider cost-effectiveness, societal value, and how the product compares to existing treatments in real-world settings.
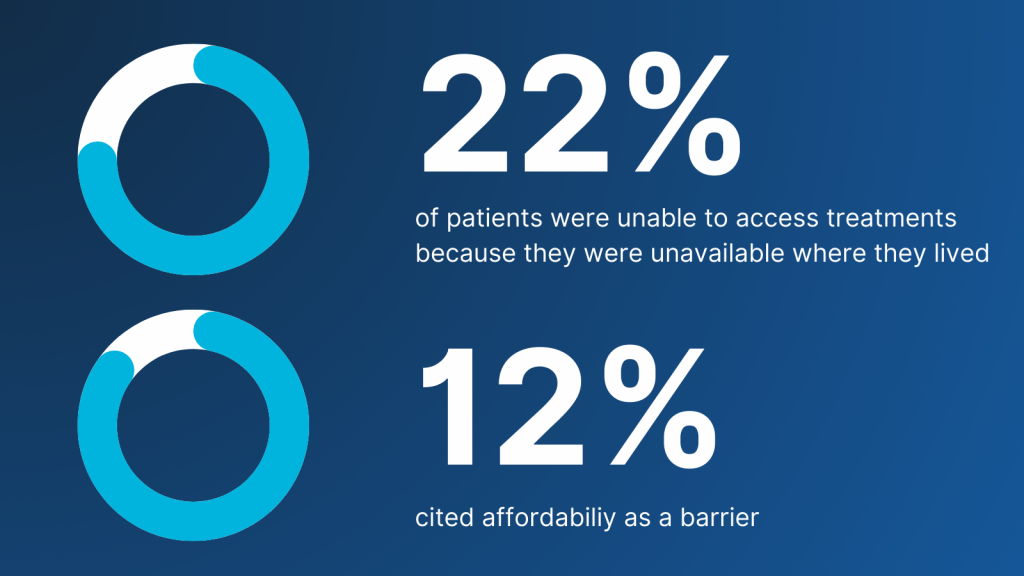
For rare disease patients, HTAs are critical. They guide decisions that expand access to life-changing therapies. Yet, 94% of rare diseases lack a specific treatment, and one-third of rare disease patients have never received therapy directly linked to their condition[i]. Even when treatments exist, 22% of patients in 2019 reported being unable to access them because they were unavailable where they lived, and 12% cited affordability as a barrier[ii].
The EU HTA Regulation (EU) 2021/2282 aims to address these inequities by harmonising HTA processes across Member States, enabling faster, fairer, and more collaborative assessments.
The HTA Regulation offers a historic opportunity to start aligning national HTA processes, and reduce national delays in decision-making for reimbursement and pricing. By ensuring transparency and involving patient voices, we should have a framework that everyone can trust.
François Houÿez, EURORDIS Director of Treatment Information and Access
A new era for Health Technology Assessment
Currently, HTA processes in the EU are fragmented. Each Member State conducts its own evaluations, leading to duplicated efforts, inconsistent outcomes, and delays in patient access to innovative therapies.
The introduction of Joint Clinical Assessments (JCAs) under the new HTA Regulation will change this dynamic, streamlining the HTA process across the European Union, although some competences (such as the decision on whether or not to reimburse a new technology) will remain a national prerogative. JCAs are EU-wide evaluations of specifically the clinical value of new treatments.
From January 2025, JCAs will be mandatory for:
- New oncology medicines
- Advanced therapy medicinal products (ATMPs)
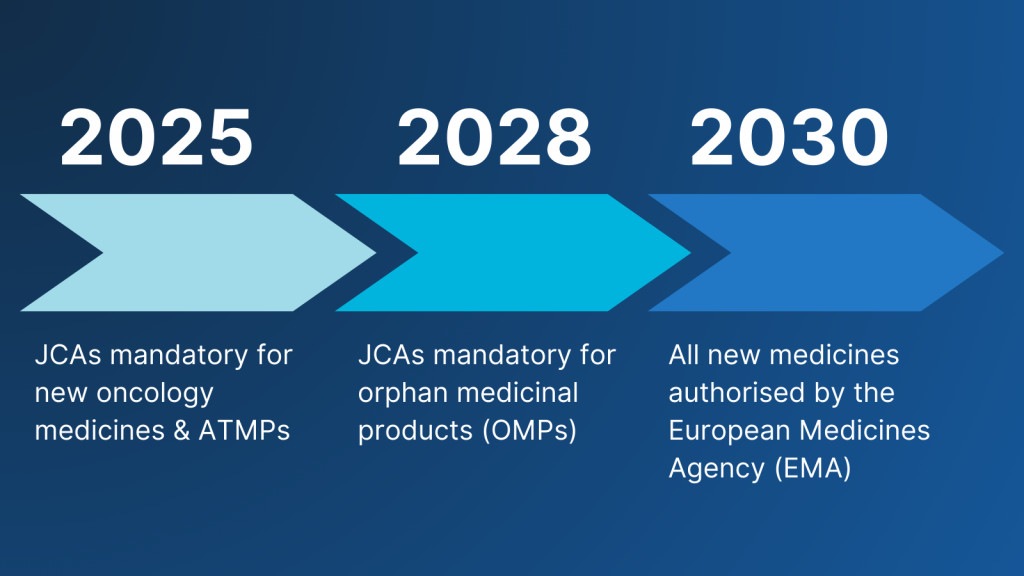
Over time, the scope will expand to include orphan medicinal products (from 2028) and all new medicines authorised by the European Medicines Agency (EMA) (from 2030). These assessments will provide a single, harmonised evaluation that Member States can use for national pricing and reimbursement decisions.
JCAs should be a game-changer for delivering faster, fairer access to life-saving therapies across Europe. By streamlining assessments and harmonising processes, they shall break down barriers that have long delayed innovative treatments from reaching the patients who need them most.
Julien Delaye, Patient Engagement Manager at EURORDIS
Key changes coming in 2025
The EU HTA Regulation will introduce significant updates, including:
- Faster and more coordinated assessments
JCAs will partly replace separate evaluations in each Member State, aligning with EMA timelines to ensure reports are published soon after a treatment is approved. Member States will have to include the European conclusions in their own national report, although these European conclusions will not be binding regarding national reimbursement procedures.
- Greater transparency
The process will become more open, with clear summaries encompassing stakeholders’ inputs of JCA reports for patients and stakeholders.
- More input from patients and experts
Patients and healthcare professionals will play a stronger role, contributing to JCA and Joint Scientific Consultations (JSCs).
- Consistent methods across Europe
Harmonised procedures will make assessments consistent and comparable across Member States, promoting equity in decision-making.
- Reduced duplication of effort
Centralising assessments at the EU level will reduce repetitive work, allowing Member States to adapt findings to their local healthcare systems.
For patients, these changes mean:
- Accelerated access to life-saving treatments.
- Enhanced fairness through standardised methodologies.
- A central role for patients, with their perspectives integrated into assessments.
From a patient advocacy perspective, the emphasis on joint clinical assessments is crucial. For too long, fragmented processes have delayed access to vital treatments. This regulation brings us closer to a unified approach that puts patients first.
Johan de Graaf, President of the Dutch Pituitary Foundation, and member of EURORDIS’ Health Technology Assessment Task Force
Challenges on the horizon and looking ahead
Despite its potential, the HTA Regulation presents challenges that must be addressed:
- National systems’ adaptation: Member States need to update their HTA processes to incorporate JCA findings effectively.
- Tight deadlines: Developers may struggle to submit high-quality data within the required timelines.
- Complexity: JCAs require extensive documentation and coordination, demanding significant expertise and resources.
EURORDIS is actively engaging with EU institutions and Member States to guarantee these challenges are successfully tackled and to facilitate smooth implementation.
Ultimately, the implementation of the HTA Regulation represents a major milestone for European healthcare. By fostering collaboration, efficiency, and patient-centred decision-making, this framework promises to improve access to innovative therapies and deliver better health outcomes for patients across Europe.
As the regulation comes into force, EURORDIS will continue to advocate for meaningful patient involvement, transparency, and alignment between EU and national systems.
Together, we can make sure the changes introduced in 2025 deliver on their fundamental promise: to create a healthcare system that is inclusive, equitable, and responsive to the needs of all patients, particularly those with rare diseases.
Julien Poulain, Communications Manager
[i] https://www.eurordis.org/our-priorities/treatments/
[ii] Ibid.
- See also: Demystifying HTA: patients’ role in new legislation (June 2022)
